
Implant related infections are the most common cause of joint arthroplasty failure and results in costs of over $8.5 billion in the United States alone, not to mention deaths and pain and suffering.
Bacteria colonize the implants’ surface and there is no blood flow in the areas surrounding the implant and the antibiotics in the blood cannot reach these areas.
Most solutions are iodine, silver ion and nano-silver coatings. Many of these coatings have shelf lives and in general these treatments have not shown significant decreases in infection rates.
Implant related infections are the most common cause of joint arthroplasty failure and results in costs of over $8.5 billion in the United States alone, not to mention deaths and pain and suffering.
Bacteria colonize the implants’ surface and there is no blood flow in the areas surrounding the implant and the antibiotics in the blood cannot reach these areas.
Most solutions are iodine, silver ion and nano-silver coatings. Many of these coatings have shelf lives and in general these treatments have not shown significant decreases in infection rates.
In many infections, bacteria form a biofilm on the implant, increasing their resistance to antibiotics and resulting in infection. Infections often persist despite aggressive surgical debridement and prolonged antibiotic treatments.
In the United States, orthopedic implants are associated with an approximate 4-12% infection rate, representing at least 100,000 infections per year.
It is estimated that implant infections increase the overall cost of hospitalization up to 45% on average.
In many infections, bacteria form a biofilm on the implant, increasing their resistance to antibiotics and resulting in infection. Infections often persist despite aggressive surgical debridement and prolonged antibiotic treatments.
In the United States, orthopedic implants are associated with an approximate 4-12% infection rate, representing at least 100,000 infections per year.
It is estimated that implant infections increase the overall cost of hospitalization up to 45% on average.
The orthopedic implants segment is expected to hold a significant medical implants market share in the global market. It is anticipated to continue this trend throughout the forecast period, owing to the rise in the prevalence of osteoporosis among the aging population and increased physical illnesses such as obesity.
The orthopedic implants segment is expected to hold a significant medical implants market share in the global market. It is anticipated to continue this trend throughout the forecast period, owing to the rise in the prevalence of osteoporosis among the aging population and increased physical illnesses such as obesity.
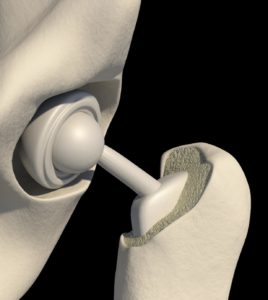
The orthopedic implants segment is expected to hold a significant medical implants market share in the global market. It is anticipated to continue this trend throughout the forecast period, owing to the rise in the prevalence of osteoporosis among the aging population and increased physical illnesses such as obesity.
6,146 hospitals in USA.
920,000 total staffed beds in all US hospitals.
224,720 total operating rooms in the United States.
56,000 surgical intensive care beds used for orthopedic surgery procedures.
7.2m+ patients were diagnosed with an orthopedic injury during the last five years.
1.2 million patients with over 1.7 million hip and knee replacement procedures.
4.9% CAGR (2017-2022) in the number of orthopedic surgery procedures per year.
28.3m orthopedic surgery procedures globally making this one of the most rapidly growing surgical procedure categories.
45% is the total knee arthroplasty orthopedic surgery lifetime risk of developing symptomatic knee osteoarthritis.
$49,360 total average cost of care for this surgery.
600,000 total knee replacements annually in the US.
$29Bn in US knee arthroplasty orthopedic procedures.
Today orthopedic issues are the most common reason people seek medical care
with 1 in 7 Americans reporting an orthopedic impairment.
“MicrobeProof is coupling UV polymetric coatings with a cost effective device in a portable drug delivery system for the operating room to personalize drug treatments at the point of care”
– Mark Raymond

The solution to treating every joint replacement with effective antibiotics is a point of care (in operating room) modular, portable device that coats the implant, screw, pin, or device with a lasting antibacterial coating that can be customized by the physician with chosen antibiotics and lasts several weeks in the body as an infection deterrent*. This solution is the first ever point of care unit to be developed for this application.
*This equipment is being developed based upon the work done by:
and shows exceptional efficacy in preventing infection.

The solution to treating every joint replacement with effective antibiotics is a point of care (in operating room) modular, portable device that coats the implant, screw, pin, or device with a lasting antibacterial coating that can be customized by the physician with chosen antibiotics and lasts several weeks in the body as an infection deterrent*. This solution is the first ever point of care unit to be developed for this application.
*This equipment is being developed based upon the work done by:
and shows exceptional efficacy in preventing infection.

The solution to treating every joint replacement with effective antibiotics is a point of care (in operating room) modular, portable device that coats the implant, screw, pin, or device with a lasting antibacterial coating that can be customized by the physician with chosen antibiotics and lasts several weeks in the body as an infection deterrent*. This solution is the first ever point of care unit to be developed for this application.
*This equipment is being developed based upon the work done by:
and shows exceptional efficacy in preventing infection.
The equipment, servicing, support for this solution represents a multi-billion-dollar opportunity in the United States, and throughout the world. While the work the team of universities achieved is stellar, this equipment and system for applying the coatings is the first of its kind and does not yet exist in the marketplace.
The equipment, servicing, support for this solution represents a multi-billion-dollar opportunity in the United States, and throughout the world. While the work the team of universities achieved is stellar, this equipment and system for applying the coatings is the first of its kind and does not yet exist in the marketplace.

Copyright © 2021, All Rights Reserved.